Diabetes–SGLT2 Inhibitor
Diabetes is a leading global health concern with an estimated 463 million adults afflicted with this disease. According to the International Diabetes Federation, this number is expected to grow to 700 million by 2045. Moreover, the cost of diabetes was USD 760 billion dollars a year in 2019.
Most diabetics treat their disease by controlling insulin production or regulating blood sugar through the use of medication. A diabetic patient can require up to 10 different medications at a time. Despite this large number of therapeutic options many diabetics are unable to control their disease and face significant health risks.
Sirona Biochem’s SGLT2 Inhibitor
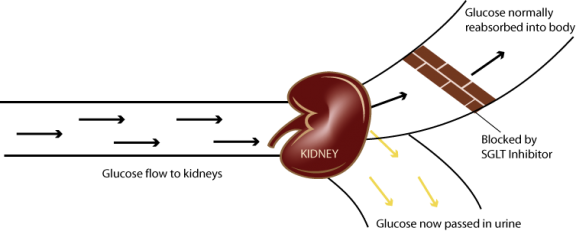
SGLT2 Inhibitors work differently from other, non-insulin, diabetes therapeutics which increase insulin production in the pancreas and/or affect metabolism. SGLT2 Inhibitors act in the kidneys to reduce the re-absorption of glucose into the bloodstream. SGLT2 Inhibitors also have the potential to be strong add-on therapies to current diabetes treatments.
Using proprietary technology, Sirona has developed an SGLT2 Inhibitor (TFC-039) with optimal pharmaceutical characteristics including, but not limited to, enhanced stability, bioavailability, selectivity and/or efficacy.
The first SGLT2 inhibitor to receive market approval in the U.S. was Johnson and Johnson’s SGLT inhibitor, Invokana™ (canagliflozin). Forxiga™ (dapagliflozin), an SGLT2 inhibitor developed by AstraZeneca and Bristol-Myers Squibb received European market approval in November 2012 and was approved by the Food and Drug Administration (FDA) for the US market in January 2014.
In April, 2019, Invokana was shown to stall the progression of chronic kidney disease as well as cardiovascular problems. SGLT2 inhibitors are being further studied for these indications.
SGLT2 Inhibitor Results
Several studies have been conducted to demonstrate the effectiveness and stability of Sirona Biochem’s SGLT2 Inhibitor SBM-TFC-039.
- SBM-TFC-039 triggers glucosuria in a dose-dependent manner
- In a glucose challenge, SBM-TFC-039 reduced blood glucose excursions by 34%
- SBM-TFC-039 reduced blood glucose in obese diabetic rats to a level of lean rats within 6 hours
- In a 28-day chronic study, SBM-TFC-039 reduced blood glucose levels in obese diabetic rats by 71%
- SBM-TFC-039 reduced blood glucose by 44% in diabetic rats compared to canagliflozin at 26%
- At 36 & 48 hours after treatment, SBM-TFC-039, at a dose of 1.0mg/kg was still effective at reducing blood glucose; canagliflozin lost its effect after 36 hours.
In head-to-head preclinical studies, Sirona Biochem’s SBM-TFC-039 outperformed Johnson and Johnson’s canagliflozin.
Commercialization
In January, 2014, Sirona announced a license agreement for its SGLT2 Inhibitor with Wanbang Biopharmaceuticals in China. Wanbang will develop and commercialize Sirona’s anti-diabetic SGLT2 inhibitor exclusively in the People’s Republic of China (PRC). In exchange for this license, Wanbang Biopharma has provided an upfront and will recieve milestone payments of up to US$9.5M in addition to royalty payments for product sales in the PRC. During development, Sirona received $1.5 Million USD from Wanbang. Wanbang completed Phase I clinical trials. In December 2021, it was announced that Wanbang would no longer be developing the product due to the launch of a generic version in China. Sirona and Wanbang continue to work together to develop the product for animal health as well as for various regions and indications.
Sirona Biochem’s SGLT2 Inhibitor is currently available for agreements such as:
- Licensing Agreements
- Royalty Agreements
- Acquisition of our technology
- Joint ventures
For any additional information please contact info@sironabiochem.com.